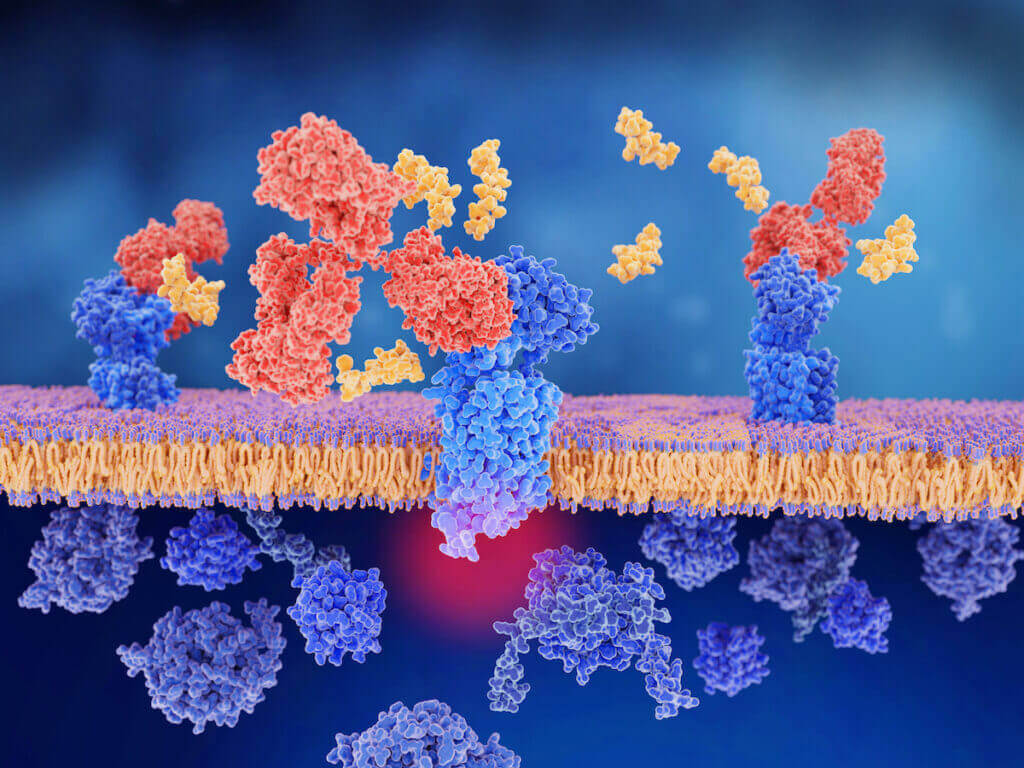
What is CGRP (Calcitonin Gene Related Peptide)?
During a migraine attack, the trigeminal nerves release a variety of inflammatory proteins. One of the main proteins is called CGRP (calcitonin gene related peptide). CGRP has been studied since the early 1980s when it was discovered. It was found throughout the trigeminovascular system and trigeminal cranial nerves which transmit pain in the head and face. Therefore, a role in migraine was suspected. The trigeminal nerves are central to causing migraine as explained here. The trigeminal nerves and their associated electrical circuitry throughout the brain, brainstem, and arteries in the brain is called the trigeminovascular system. This system is the basis and an “on switch” for migraine.
In the early 1990s it was shown that CGRP was released by the trigeminal nerves and levels increased during an acute migraine attack. In 2004, a CGRP antagonist (blocks the binding of CGRP to its receptor) was shown to abort (stop) an acute migraine attack, and decrease CGRP levels. Subsequent preventive migraine studies done since 2014 with a CGRP antibody to block the effects of CGRP continued to show reduction in migraine frequency and severity.
CGRP causes inflammation around the brain and cerebral arteries (“sterile inflammation”) in the dural membrane surrounding the brain, intensifies pain signals, enhances transmission of pain signals through the trigeminal nerves into the brainstem and into the brain, and causes dilation of the cerebral arteries through the dural membrane. Trigeminal nerve endings surround these arteries. The dilation of these arteries triggers these trigeminal nerve endings and this leads to further increasing pain signals. Think of the process like a painful meningitis, minus the infection.
The result of these steps is intense migraine pain (as you are unfortunately very familiar with). So, if we can block these steps of migraine pain, the attack should be aborted quickly, and not as severe. That’s the thinking here, and that’s where the CGRP medications (gepants and CGRP monoclonal antibodies) come into play, as discussed below.
CGRP MEDICATIONS USED TO ABORT MIGRAINE (TAKEN AS NEEDED)
Abortive migraine medications are medications taken at the onset of the migraine with a goal of lessening the duration and severity of the migraine attack. Historically, the options have included NSAIDs, ergots, triptans, and neuromodulatory devices.
The gepants were the first new medicine class to emerge as new migraine abortive options (FINALLY!!!) since the triptans became available in 1992. There are currently 2 oral pill gepant abortive options available ((Ubrelvy (Ubrogepant), Nurtec ODT (Rimegepant)) and one nasal spray abortive (Zavspret (Zavegepant). These 3 gepants are discussed and compared in much greater detail here.
How do the gepants (Nurtec, Ubrelvy, Zavspret) work?
Gepants work as CGRP receptor antagonists, which means they directly block (antagonist) the CGRP receptor. This results in the medication “blocking” the CGRP inflammatory protein from sticking to the CGRP receptor to activate it, and thus prevents it from “turning on” the pathways of pain described above.
So, you get reversal of cerebral vasodilation, which decreases the firing off of the trigeminal nerves. Notably, the gepants do this in a way that does not cause vasconstriction, in contrast to the triptans. Thus, they are felt to be safe in those with cardiovascular or cerebrovascular disease (as opposed to the triptans).
By blocking the CGRP receptor, you also get reversal of the neurogenic inflammation going on through the brain and around the arteries, and you block the electrical transmission of migraine pain from traveling from the trigeminal nerves into the brainstem, and ultimately into the brain.
The other huge benefit of the triptans compared to all other abortive options is that they do not cause medication overuse headache (rebound headache)!
What are the gepant side effects (Nurtec, Ubrelvy, Zavspret)?
The side effect profile of the gepants is minimal and similar to placebo. The most common side effects of gepants are very low risk of nausea for Nurtec ODT and low risk of nausea and mild sedation with the higher dose of Ubrelvy. Side effects are discussed in more detail here.
In addition, there is no interaction with using them and triptans, NSAIDs, or other acute meds in case they happen to be taken close together.
Compared to other abortive medications such as the triptans and NSAIDS, these medications are not associated with medication overuse headache (rebound headache), which is great! They also have no addiction potential.
Compared to the triptans and ergots, these medications are NOT contraindicated in patients with stable cardiovascular or peripheral vascular disease or risk factors because they do not cause vasoconstriction (narrowing) of the arteries, which is a HUGE benefit.
Triptans are also contraindicated in patients with visual snow, persistent migraine aura, and migrainous stroke (infarction). However, gepants are felt to be safe for these patients, as well as those with hemiplegic migraine and migraine with brainstem aura (previously called basilar migraine). There are many patients who have been stuck without safe options since they have been unable to use standard therapies such as triptans due to other medical problems such as heart disease. So, we finally have a safe alternative for them, which is a highlight of these medications.
Can the gepants (Nurtec, Ubrelvy, Zavspret) be used in pregnancy and breastfeeding?
Safety of these medications in pregnancy or breastfeeding is unknown because they haven’t been studied, and therefore are not recommended. There was one study which showed that the amount of Nurtec that enters the breastmilk is a very small fraction, felt to likely be negligible. Regardless, further data is needed before confirming safety.
Are there drug interactions with the gepants (Nurtec, Ubrelvy, Zavspret) and other medications?
The primary drug interactions to be aware of with these medications are when used with other medications that are metabolized by the liver enzyme system called CYP3A4. Many commonly used medications are metabolized by this system. Strong or moderate inhibitors of CYP3A4 (which slow down the metabolic drug breakdown) will cause an increase in gepant blood levels. Strong or moderate inducers of CYP3A4 (which increase the metabolic drug breakdown) will cause a decrease in gepant blood levels and possibly decreased effectiveness. These medications should be avoided in patients with severe liver disease or end stage kidney disease such as those on dialysis.
CGRP MEDICATIONS USED TO PREVENT MIGRAINE
Preventive migraine treatments are used to lessen the frequency and/or severity of migraine attacks. Preventive treatments include a variety of daily pill medications, neuromodulatory devices, herbal and natural supplements and vitamins, yoga and meditation, acupuncture and acupressure. All of the medications used for migraine prevention have always been “adopted” from other specialties. In other words, these were medicines made for other purposes (such as antidepressants, antiseizure, anti-blood pressure meds), but eventually some were also found to be useful for migraine prevention.
There has never been a medicine engineered and created purely and only for migraine prevention. However, that changed in 2018 when the migraine preventive landscape changed abruptly and significantly. The 1st medication class designed purely and only for migraine prevention become available, called the CGRP monoclonal antibodies (mAbs). There are currently 4 CGRP mAb treatment options. They are Aimovig (Erenumab), Emgality (Galcanezumab), Ajovy (Fremenazumab), and Vyepti (Eptinezumab).
These medications either target the CGRP receptor (Aimovig), or the CGRP protein (Emgality, Ajovy, Vyepti). The result of “blocking” the CGRP protein or CGRP receptor prevents the CGRP pathways of pain from “turning on”, as discussed above and here. Clinically, some patients tend to respond better to the CGRP receptor blockade, whereas others tend to do better with binding the CGRP protein itself. There is not really any data on this in terms of who may respond to which type of CGRP mAb target, but I’m sure it will be studied further eventually.
Aimovig, Emgality, and Ajovy are all once monthly self-injections (push button autoinjection), although Ajovy also has the option of quarterly injections (3 injections every 3 months). Vyepti is the only IV (intravenous form) and is done by 30-minute IV treatment every 3 months. These individual CGRP mAbs are discussed and compared in much greater detail here.
What are the side effects of the CGRP mAbs (Aimovig, Emgality, Ajovy, Vyepti)?
Compared to most other medications used for migraine prevention historically, the side effects of the CGRP mAbs are very low. The most common side effects reported (at a very low rate) are mild upper respiratory infections and minor injection site reactions. Aimovig has a slightly increased risk of constipation and possible mild increase in high blood pressure for some patients. These have little to no drug interactions and do not affect the liver or kidneys. Data show no immunological (they do not suppress or alter the immune system because they do not have a target within the immune system), cardiovascular, or neurological safety concerns of significance.
Can the CGRP mAbs (Aimovig, Emgality, Ajovy, Vyepti) be used in pregnancy and breastfeeding?
There is no data to answer this question yet. However, CGRP is suspected to play a possible role in regulating uteroplacental blood flow, myometrial and uterine relaxation, and in maintaining normal gestational blood pressure. Since the mAbs have a long half-life and can last in the system for 5 months, it is recommended to stop it about 6 months prior to pregnancy planning. The CGRP mAbs are also not recommended to use during breast-feeding since we do not have enough safety data at this time.
Nurtec and Qulipta (gepants) for migraine prevention.
The CGRP mAbs have been a major step forward for migraine prevention. However, up to this point, we still have not had an oral pill that has been engineered, created, and researched purely and only for migraine (not “adopted” from a different medicine class as mentioned above).
Then, on 5/27/21, Nurtec ODT (Rimegepant) made history as the first and only FDA approved medication for BOTH abortive and preventive migraine treatment simultaneously, and the only option with this flexibility and is discussed in greater detail here!
The perspective behind this is that migraine is a fluid and variable disease, fluctuating between periods of episodic migraine (1-14 headache days per month), and other periods of chronic migraine (15 or more headache days per month). So, having a medicine that can function as both types of treatment, depending on what type of phase the migraine is in (episodic or chronic) opens up an entirely new flexible treatment paradigm and approach which we have never had up to this point.
So essentially, taking Nurtec ODT every other day could be used as an ongoing daily preventive strategy (the long half-life of 11 hours allows for this spread-out dosing) when the migraine is in a high frequency to chronic migraine phase. If it evolves back into a lower frequency episodic migraine pattern, it can then just be used abortively only when needed for a migraine attack.
This new flexible dosing option of Nurtec ODT could also be used as a “mini-prophylaxis” within the month. For example, if patients know they are approaching a predictable migraine trigger, such as menstrual migraine, barometric trigger from an airplane trip, upcoming stressful event such as an exam, etc., the medication could possibly be taken daily or every other day starting a few days before the anticipated trigger, and stopping it a day or so after the trigger is no longer present. Unlike other migraine preventive pill treatments which take 4-6 weeks to start working and 2-3 months to see full effect, the gepants work fast and this would allow this potential treatment option to begin working immediately. In fact, studies show that migraine frequency dropped by 30% within the first week alone of preventive use.
Atogepant (Qulipta) is the 2nd gepant that was FDA approved as a migraine preventive pill in September 2021. It is the first gepant that was both developed and studied purely for the prevention of migraine (not used abortively).
In April 2023, it was FDA approved for chronic migraine as well. So at this point, Qulipta is the only oral gepant which is approved for both episodic and chronic migraine.
Can the preventive CGRP mAbs (Aimovig, Emgality, Ajovy, Vyepti) be used with the CGRP abortive gepant medications (Nurtec ODT, Ubrelvy, Zavspret)?
Can I use Aimovig with Nurtec ODT? Can I use Aimovig with Ubrelvy? Can I use Emgality with Nurtec ODT? Can I use Emgality with Ubrelvy? Can I use Ajovy with Nurtec ODT? Can I use Ajovy with Ubrelvy? Can I use Vyepti with Nurtec ODT? Can I use Vyepti with Ubrelvy? These are very common questions. Unfortunately, there aren’t many studies so far to clarify this, although I’m sure these questions will be studied and clarified in the near future. The gepants and the CGRP mAbs have much different structures, molecule sizes, and metabolism.
So theoretically, it would make sense that using an abortive CGRP medication (gepant) on top of a CGRP preventive medication (CGRP mAb) would give synergistic (working together) benefit. Using a CGRP preventive medication targeting the CGRP protein (Emgality, Ajovy, Vyepti) and a CGRP abortive medication targeting the CGRP receptor (Nurtec ODT, Ubrelvy) seems like a very sensible idea. Similarly, using a CGRP preventive medication targeting the CGRP receptor (Aimovig) combined with a CGRP abortive medication also targeting the CGRP receptor (Nurtec ODT, Ubrelvy) would make a lot of sense too. In fact, there are some limited studies which provide evidence that these medications used together do work better and are safe.
There was a publication of data from only a 2-patient cohort showing that the use of these acute and preventive CGRP migraine therapies together can be successful and safe. These two patients had been using Rimegepant (Nurtec ODT) in a long-term safety study and they had added Erenumab (Aimovig) once monthly injection as a preventive treatment. After Aimovig was added, patient 1 had 100% relief for 7 of 7 acute migraine attacks treated with Nurtec. Patient 2 had 100% relief for 9 of 9 acute migraine attacks treated with Nurtec. So, the combination of using Nurtec abortively in addition to using Aimovig preventively appeared to provide an even more effective acute migraine response. Larger studies to confirm the suspicion that they likely work together synergistically will be helpful.
There was a larger safety study publication which evaluated the acute treatment of migraine with Rimegepant while using a CGRP monoclonal antibody for the prevention of migraine. The CGRP mAbs used were Erenumab (Aimovig) (7 patients), Fremanezumab (Ajovy) (4 patients), and Galcanezumab (Emgality) (2 patients). The study determined that Rimegepant used as an acute migraine treatment in combination with CGRP mAbs for migraine prevention was well tolerated with no safety issues identified. The researchers concluded that the probability between these 2 classes (gepants and CGRP mAbs) was low, especially because they have entirely different pathways of drug metabolism. The gepants are metabolized in the liver, while the CGRP mAbs are metabolized and cleared in the reticuloendothelial system. They also concluded that existing evidence supports the safety of combined use, although further larger research was warranted.
Can the CGRP mAbs (Aimovig, Emgality, Ajovy, Vyepti) be used with Botox (Onabotulinumtoxin A) for chronic migraine?
The answer is yes. Insurance companies often present various hurdles to using preferred treatment options (the bane of my existence). One common issue for patients with chronic migraine who are receiving Botox injections is that most insurance companies will now make the patient choose between Botox or the CGRP mAb. There is of course no good scientific basis for this, other than the company doesn’t want to pay for both.
Actually, there is evidence that using Botox with the CGRP mAbs works better together than with either individually. An abstract presented at the American Headache Society Annual Scientific meeting in June 2020 showed that in patients with chronic migraine and a baseline frequency of 25.7 days per month, the frequency dropped to 14.8 days with Botox, and 9.1 days with Botox plus a CGRP mAb.
Can I still use my CGRP mAb (Aimovig, Ajovy, Emgality, Vyepti) with the Covid-19 vaccine?
This hasn’t been a reported issue thus far. There is no current evidence for an interaction between the Covid-19 vaccine and CGRP mAbs, the same as any other vaccine. This has also been stated by the American Migraine Foundation. Patients receiving CGRP mAbs were not excluded from the Covid-19 vaccine trials. There is no evidence at this time that these treatments cannot be used along with receiving Covid-19 vaccination, nor do they need to be delayed or timed any differently in relation to receiving Covid-19 vaccination.
Most physicians feel that there should theoretically be no interaction or contraindication to receiving either of these treatments in relation to Covid-19 vaccination because they are entirely different proteins with different mechanisms of action. The Covid-19 vaccine stimulates the immune system to form antibodies against the virus, should you encounter it. The CGRP mAbs do not have any significant influence on the immune system (they do not cause immunosuppression, etc.).
Rarely, the immune system of some patients can form neutralizing antibodies against the CGRP mAbs, and this can weaken the effectiveness of these treatments in their ability to decrease migraine frequency and severity. However, this rarity really has nothing to do with the mechanism and how the Covid-19 vaccine works. So, it is not felt that the Covid-19 vaccine will lessen the effectiveness of these treatments, nor will these treatments lessen the effectiveness of the Covid-19 vaccine.
Notably, there have been just a few isolated reports of dermal fillers used in dermatology causing some facial swelling in association with Covid-19 vaccination, but not with Botox or the CGRP mAbs. These reports were with the Moderna Covid vaccine and resolved with steroids and/or antihistamines. The topic of Covid-19 headache and Covid-19 vaccination is discussed further here.
IF YOU HAVE HEADACHE, MIGRAINE, OR FACIAL PAIN AND ARE LOOKING FOR ANSWERS ON ANYTHING RELATED TO IT, A HEADACHE SPECIALIST IS HERE TO HELP, FOR FREE!
FIRST, LET’S DECIDE WHERE TO START:
IF YOU HAVE AN EXISTING HEADACHE, MIGRAINE, OR FACIAL PAIN DIAGNOSIS AND ARE LOOKING FOR THE LATEST INFORMATION, HOT TOPICS, AND TREATMENT TIPS, VISIT OUR FREE BLOG OF HOT TOPICS AND HEADACHE TIPS HERE. THIS IS WHERE I WRITE AND CONDENSE A BROAD VARIETY OF COMMON AND COMPLEX MIGRAINE AND HEADACHE RELATED TOPICS INTO THE IMPORTANT FACTS AND HIGHLIGHTS YOU NEED TO KNOW, ALONG WITH PROVIDING FIRST HAND CLINICAL EXPERIENCE FROM THE PERSPECTIVE OF A HEADACHE SPECIALIST.
IF YOU DON’T HAVE AN EXISTING HEADACHE, MIGRAINE, OR FACIAL PAIN DIAGNOSIS AND ARE LOOKING FOR POSSIBLE TYPES OF HEADACHES OR FACIAL PAINS BASED ON YOUR SYMPTOMS, USE THE FREE HEADACHE AND FACIAL PAIN SYMPTOM CHECKER TOOL DEVELOPED BY A HEADACHE SPECIALIST NEUROLOGIST HERE!
IF YOU HAVE AN EXISTING HEADACHE, MIGRAINE, OR FACIAL PAIN DIAGNOSIS AND ARE LOOKING FOR FURTHER EDUCATION AND SELF-RESEARCH ON YOUR DIAGNOSIS, VISIT OUR FREE EDUCATION CENTER HERE.