
Can Migraines Cause Dizziness Without Pain?
Have you ever experienced dizziness without the classic throbbing pain of migraine headaches? Can migraines cause dizziness without


Have you ever experienced dizziness without the classic throbbing pain of migraine headaches? Can migraines cause dizziness without

I see patients stuck in the dark lonely rut of chronic migraine all day every day. Chronic migraine

Are you tired of relying on traditional prescription medications to manage your migraine headaches, or do you get

For many people living with fibromyalgia, the battle against chronic body pain is an everyday fight. There is

Do you find yourself searching for effective natural relief of debilitating headaches and migraines that doesn’t involve relying

High altitude headache, also known as altitude headache, commonly affects people who ascend to higher elevations, typically more

Are you dreading the wildfire smoke season lighting up your migraines again this year? Are you experiencing frequent

Caffeine and migraine, what’s the connection? While caffeine is often touted as a remedy for headaches because it
Optical migraine Have you ever experienced strange visual disturbances in your field of vision that come out of
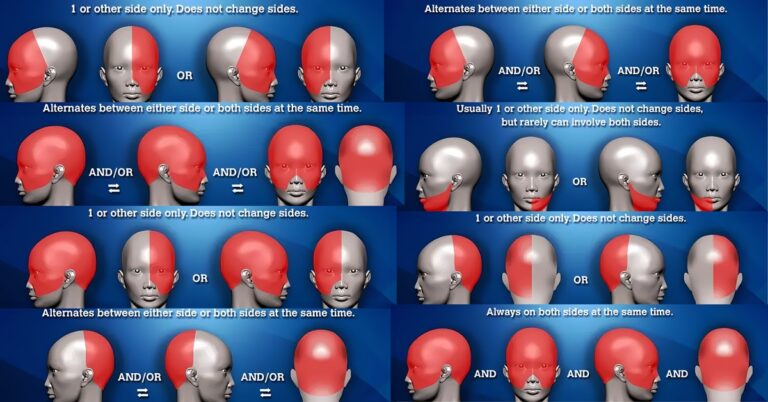
Headache placement meanings and headache location meanings. These are common questions patients search for online (usually anxiously and

When it comes to managing headaches and migraines, a comprehensive approach is key. Accurate diagnosis and effective treatment

Battling hunger headaches can be frustrating, but with the right approach, it’s manageable. How to cure hunger headaches
Please send your positive/negative feedback, success stories, suggestions, advertising inquiries, partnerships, or other comments.