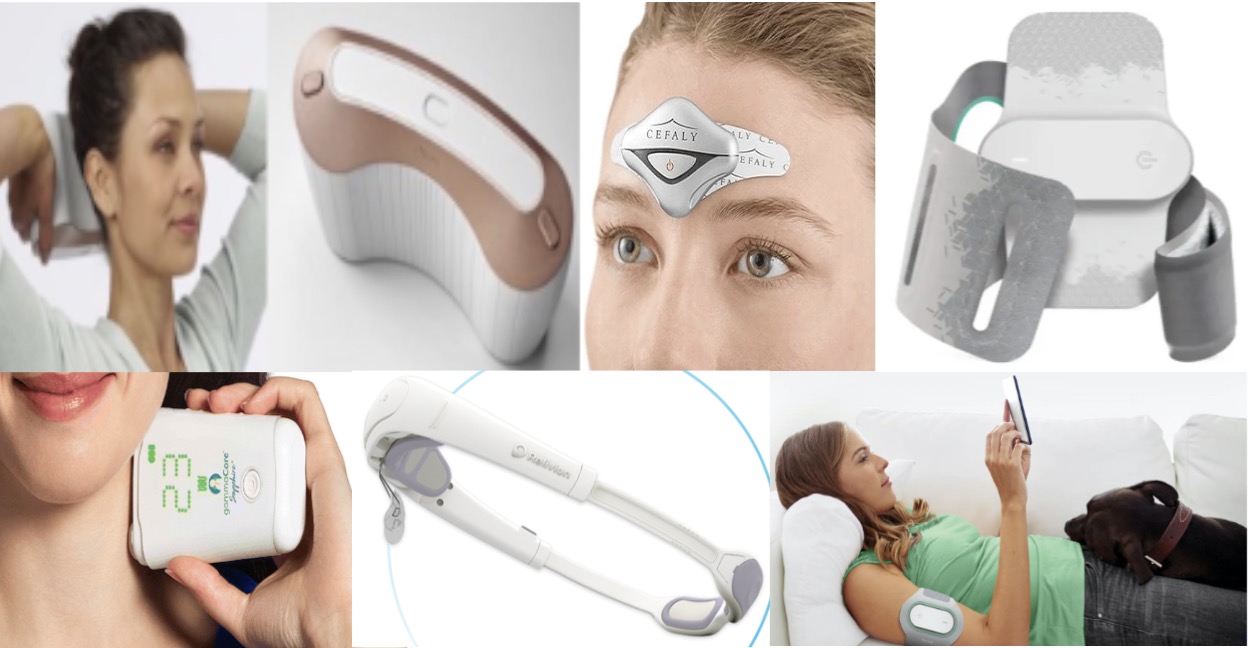
What are the neuromodulation devices available for migraine headache treatment, and what are the differences?
There are 5 main migraine neuromodulation devices available. They are handheld or wearable devices and include Nerivio, GammaCore Vagus Nerve Stimulator, SAVI Dual, Cefaly, and Relivion MG. All 5 of them are FDA cleared for abortive (acute) migraine treatment. All of them are also FDA cleared for preventive migraine treatment, with the exception of Relivion MG. GammaCore is unique in that it is also FDA cleared for the treatment of cluster headache, hemicrania continua, and paroxysmal hemicrania.
There are a few different spin-off type neuromodulation devices on the market that can be purchased directly online without a prescription. The main difference to be aware of is that these don’t have the rigorous clinical trials including placebo (sham) arms, nor FDA clearances.
The first of these non-prescription devices is the Truvaga vagus nerve stimulator. Truvaga is made by the same company which makes GammaCore vagus nerve stimulator and uses the same technology, but without a prescription. It is detailed more under the vagus nerve stimulation section below.
ZOK and the Migracorr Migraine Stopper are two different devices that were each created by a chiropractor rather than a physician. These two devices use an ear piece in one ear to induce pressure changes and reportedly stimulate the trigeminal and vagus nerves to treat headache, although there are no robust scientific clinical studies to confirm this, nor efficacy studies. Nocira is another device which is inserted into both ears to provide puffs of air into the ears with the same theoretical mechanism of action. A pilot clinical study evaluating Nocira for a single migraine episode in 30 patients reported that 67% of the study patients were pain-free in less than 20 minutes. A more rigorous scientific study is reportedly being planned according to the website.
What is neuromodulation?
Migraine is an electrical event that occurs in the brain. Think of your brain immersed in an electrical storm during a migraine. Then think of neuromodulation devices like a reset button, as if you are rebooting a malfunctioning device. Neuromodulation devices provide a non-pill treatment option for both acute and preventive treatment of migraine, cluster headache, hemicrania continua, and paroxysmal hemicrania, as well as some additional neurological disorders such as epilepsy and depression.
When to consider neuromodulation devices for migraine
Some people do not like the idea of taking pills or medications to treat their migraines. Others may not respond to standard migraine abortive and preventive medications, may not tolerate their side effects, or cannot take them due to medical contraindications. These are all common reasons to consider a neuromodulation device. The neuromodulation devices are also commonly used as adjunctive treatments used in combination with other standard migraine treatments. Neuromodulation devices do not cause medication overuse headache (rebound headache), which is normally caused by using too much abortive migraine medicines.
What is the best neuromodulation device for migraine and cluster headache?
Cefaly vs. Nerivio, Nerivio vs. GammaCore, Cefaly vs. GammaCore, Nerivio vs. SAVI Dual, Cefaly vs. SAVI Dual, GammaCore vs. SAVI Dual, Relivion vs. Cefaly, Relivion vs. Nerivio, GammaCore vs. Relivion, SAVI Dual vs. Relivion? Which do you pick? Which is best for you? How do you know?
The full technical terms for the migraine neuromodulation devices are wireless remote electrical neuromodulation (REN) (Nerivio), Single-Pulse Transcranial Magnetic Stimulation (sTMS) (SAVI Dual; formerly SpringTMS and sTMS mini), external trigeminal nerve stimulation (eTNS) (Cefaly), noninvasive Vagus Nerve Stimulation (nVNS) (GammaCore), and external combined occipital and trigeminal neurostimulation (eCOT-NS) (Relivion MG).
We’ll discuss these devices in the order in which they became available and FDA cleared. We’ll also highlight differences that may help in choosing which one may be best for you to try first, in discussion with your doctor.
SAVI DUAL (SINGLE-PULSE TRANSCRANIAL MAGNETIC STIMULATION)
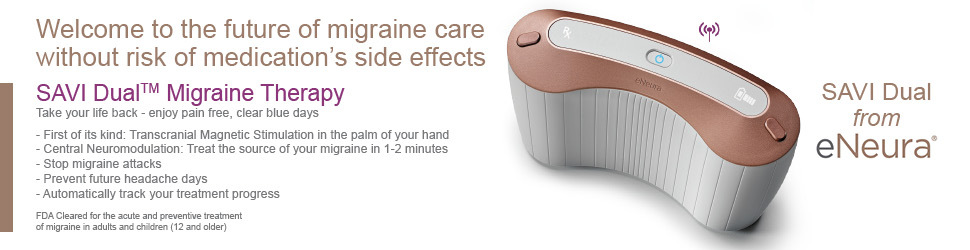
What is SAVI Dual?
Transcranial magnetic stimulation (TMS) treatments are discussed in greater detail here, and have been used for depression as well. The first neuromodulation device FDA cleared for migraine treatment was the Single-Pulse Transcranial Magnetic Stimulation (sTMS), made by the company eNeura. It was initially FDA cleared for the acute treatment of migraine in adults in December 2013. It then received additional FDA clearance for preventive treatment of migraine in adults in 2017. In February 2019, FDA clearance expanded to the acute and preventive treatment of migraine in children 12 years of age and older. The current model is called SAVI Dual. Prior models included the Spring TMS and sTMS mini.
@neuralgroover Neuromodulation Devices For Migraine Part 1: SAVI Dual… #SAVIDual #Neuromodulation #NeuromodulationForMigraine #TMS #sTMS #tmstreatment #electromagnetic #TranscranialMagneticStimulation #eNeura #Migraine #migrainerelieftok #ChronicMigraine #Migrainerelief #MigraineAbortive #migraineprevention #MigrainePreventive #Neurology #HeadacheSpecialist #Headachedoc #headachedoctor #headachemedicine #migrainesociety #migrainetok #migrainetiktok #neuroscience #neurologist #brain #braindoctor #neurologistsoftiktok #headachetiktok #medical #medicaltiktok #medicaltiktoks #MigraineWarrior #headaches #Neuro #neurologytiktok #neurotok #headache #MigraineAwareness #foryou #fyp #tiktok #foryourpage #explore #trending #health #wellness #migrainesufferer #pain #depression #anxiety #chronicpain #medicalinnovation #veteran #veterans #vasystem #veteransadministration #veteransbenefits #concussion #tbi #traumaticbraininjury #combatveteran @eneura ♬ original sound – Virtual Headache Specialist🤯
How does SAVI Dual work?
SAVI Dual delivers a single low frequency electromagnetic field that passes painlessly through bone and tissue and into the occipital cortex (visual cortex) in the back of the brain in less than 1 second. This calms that hyperexcitable electrical migraine storm in the brain and helps to shut it off. It is also theorized to stop cortical spreading depression, which is the cause of visual aura symptoms which come from the back of the brain. In fact, SAVI Dual is held across the back of the head, and this targets the cortical spreading depression of visual aura coming from the visual cortex located there.
On a side note, anecdotally, I have seen several patients with either visual snow syndrome or persistent migraine aura that I gave a treatment to in the office out of curiosity (given that these visual symptoms come from the occipital cortex), and they reported some temporary benefit. So perhaps one day they will study SAVI Dual for preventive treatment of visual snow syndrome or persistent migraine aura if we gather enough anecdotal evidence to do a study, but currently it is not FDA cleared for these specific disorders.
How is SAVI Dual used?
The user holds the device against the back of the head, and presses a button to release a very short magnetic pulse at the onset of aura or the onset of pain if they have migraine without aura.
Acute migraine treatment consists of 4 magnetic pulses at the onset of a migraine (aura or pain). Then wait 10-15 minutes. If needed, treat with an additional 4 pulses. Then wait another 10-15 minutes. If needed, treat with an additional 4 pulses. There is no limit to how many cycles can be done as needed.
Migraine prevention treatment consists of 4 pulses twice daily.
The FDA claim does not state a maximum pulse usage, and the company encourages patients to use as many pulses as needed to in order to achieve maximum relief.
How effective is SAVI Dual?
Acute treatment studies reported that 39% of patients were pain free at 2 hours. Migraine preventive studies reported that 46% of patients had a greater than 50% reduction in monthly headache days, and averaged approximately 3 less migraine days per month. All of the migraine clinical trials confirming effectiveness are outlined here.
What are the side effects of SAVI Dual?
The most common side effects reported in trials were mild and included light-headedness/dizziness, tingling over the back of the head, brief tinnitus (ringing in ears), nausea, and muscle spasm. Sometimes a tapping sensation on the scalp is described. I’ve tried it on myself and it feels like a quick puff of air to the hair on the back of the head.
Caution should be used with any kind of metal implants, similar to an MRI. If you have MRI safe implants of any kind in the head or neck, sTMS is felt to be safe to use. It is suggested that patients with implants affected by a magnetic field should not use this device. Examples of such implants include aneurysm clips or coils, cochlear implants, cerebral spinal fluid shunts, bullets or pellets lodged in the head or upper body, metal plates, screws, staples or sutures in skull, neck, shoulders, arms or hands, and facial tattoos with metallic ink. Dental implants, fillings or other dental appliances are okay to use the device.
Patients with seizures aren’t an absolute contraindication for SAVI Dual treatment, but caution is advised. The FDA listed seizure history as a “warning” in the FDA clearance label. You should not use this device if you have a cardiac pacemaker, vagus nerve stimulator (VNS) or other implanted neurostimulator, implanted cardioverter defibrillator (ICD) or any implanted medical device that stimulates the body or uses any signal from the body.
How much does SAVI Dual cost and how do I get a SAVI Dual device?
Prescribing Process:
There is a prescription form found on the company’s website which is filled out and faxed in by your doctor. The company then contacts the patient to coordinate payment and delivery. Prescriptions are processed through a specialty pharmacy as a pharmacy benefit (instead of a medical benefit). They will also help to determine if there is any insurance coverage available.
Pricing: (Pricing and promotions may vary, and updates can be found on the company’s website)
SAVI Dual costs $395/month. You can pay the cost up front for a 3 or 12 month prescription, or it can be paid in monthly installments.
The monthly prescription includes unlimited acute and preventive doses/treatments, personalized clinical specialist (RN) support throughout treatment beginning as soon as the package arrives, and smart neuromodulation technology and automated diary.
Insurance:
SAVI Dual is fully covered by VA (Veterans Administration) health care benefits. It is a covered product with an expanding list of private payers and regional health systems. Insurance coverage varies on a state by state basis, but if a patient’s particular plan is not currently covering, it may instead require a prior authorization or Letter of Medical Necessity prior to coverage. Most FSA and HSA programs cover SAVI Dual and other neuromodulation devices.
CEFALY
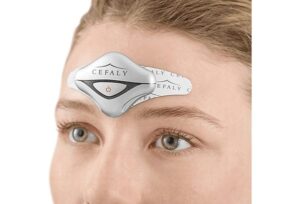
What is Cefaly?
Cefaly is made by Cefaly Technology. It was FDA cleared for preventive migraine treatment for 18 years and older in March 2014, and for acute migraine treatment for 18 years and older in November 2017. Cefaly Connected is the newest model, preceded by Cefaly Enhanced, Cefaly Dual, Cefaly Acute/Preventive, and Cefaly 1. All of the newer models have treatment settings for both acute and preventive migraine treatment.
How does Cefaly work?
Cefaly works by external trigeminal nerve stimulation (eTNS). The trigeminal nerve is the nerve that innervates throughout the brain, face, and head, and is the central component to a migraine attack. Cefaly works by sending electrical signals into the trigeminal nerve branches in the forehead to disrupt the electrical circuitry involved in a migraine attack.
How is Cefaly used?
Cefaly is applied to the forehead for 60 minutes as an acute migraine treatment. It may be repeated for a second 60-minute session if the migraine pain is not relieved within two hours, or if another migraine attack occurs. Migraine prevention treatment consists of a daily 20-minute stimulation.
How effective is Cefaly?
Migraine abortive studies reported that at 2 hours, 26% were pain free, 57% had resolution of their most bothersome migraine symptom (nausea, sensitivity to light or sound), and 43% had resolution of all migraine symptoms. Another study reported that 32% became pain free. 79% had significant pain relief with an average intensity reduction of 59% which continued up to 24 hours. Migraine preventive studies reported that 38% of patients had a 50% or more decrease in monthly migraine days. Overall, there was a 30% average reduction of monthly migraine days.
What are side effects of Cefaly?
A mild buzzing and pressure sensation are sometimes described. It should be avoided with implanted metallic or electronic devices in the head, or who have a cardiac pacemaker or implanted or wearable defibrillator.
How much does Cefaly cost and how do I get a Cefaly device?
Cefaly is available without a prescription and can be purchased on the company’s website. Pricing and promotions vary, and updates can be found on the company’s website. Currently, Cefaly costs $424 for the newest Cefaly Connected model, and $389 for the Cefaly Enhanced model. There are also bundles of devices and extra electrodes available on the website. Cefaly is fully covered by VA (Veterans Administration) health care benefits. Most FSA and HSA programs cover Cefaly and other neuromodulation devices.
VAGUS NERVE STIMULATOR (GAMMACORE)
What is GammaCore?
Noninvasive Vagus Nerve Stimulation (nVNS) is made possible by a hand-held device called GammaCore, from the company ElectroCore. The current model is GammaCore Sapphire.
GammaCore was FDA cleared for the acute treatment of cluster headache in adults in April 2017. This was followed by acute treatment of migraine in adults in January 2018, cluster headache prevention in November 2018, and migraine prevention in March 2020. In February 2021 it was FDA cleared for both abortive and preventive treatment of migraine in adolescents 12 years and older. In September 2021 it was FDA cleared for abortive and preventive treatment of hemicrania continua and paroxysmal hemicrania as well.
GammaCore the only device which is also FDA-cleared for the treatment of cluster headache, hemicrania continua, and paroxysmal hemicrania.
How does GammaCore work?
Gammacore is placed over the vagus nerve on the upper neck, just below the angle of the jaw where the pulse of the carotid is felt in the neck. If the headache is one-sided, it should be applied to the same side. GammaCore activates the vagus nerve with gentle electrical stimulation through the skin. The vagus nerve is an important highway of communication between your brain and many parts of the body. It’s sometimes referred to as the “superhighway” of the nervous system.
The vagus nerve plays an important role in regulating pain. Stimulating the vagus nerve helps blocks the pain signals causing various types of headache attacks.
When the sympathetic and parasympathetic systems are not balanced, a variety of autonomic symptoms can occur. Stimulating the vagus nerve activates the parasympathetic nervous system (aka “rest and digest system”) and brings the systems into balance. This can alleviate a variety of autonomic symptoms, as well as headache disorders.
Notably, there is an implantable vagus nerve stimulator which has been used for many years in epilepsy and depression treatments, highlighting the wide range of neurologic influence the vagus nerve has.
How is GammaCore used?
Acute treatment of migraine, cluster headache, hemicrania continua, and paroxysmal hemicrania consists of 2 two-minute stimulations. Dosing can be repeated as needed. Preventive treatment of migraine, cluster headache, hemicrania continua, and paroxysmal hemicrania consists of 2 two-minute stimulations twice daily (morning and bedtime).
How effective is GammaCore for migraine?
Studies showed significant pain relief in as soon as 30 minutes, and reported that at 1 hour, 21% were pain free and 35.8% had significant pain relief. At 2 hours, 30.4% were pain free and 40.8% significant pain relief. GammaCore reduced pain intensity over 3x greater than sham (fake device) at 60 minutes and over 6x greater at 120 minutes, and reduced the need for other rescue medications.
Studies showed a 29% reduction in migraine days per month on average when used preventively, although this number was even higher in those with aura at a 35.8%. 45% of patients had a 50% or more decrease in migraine days per month compared to 27% in the placebo (sham) group.
How effective is GammaCore for cluster headache?
Significantly more cluster headache attacks treated with GammaCore were pain-free at 15 minutes vs. those treated with placebo (47.5% vs 6.2%) in one study. Combined study data showed that significantly more (over 2-4x greater response) episodic cluster headache patients responded (no pain or mild pain) to GammaCore at 15 minutes for 50% or more of all treated attacks vs those receiving sham (34.2-64.3% vs 14.9-15.4%). At 15 minutes, there were also significant reductions in pain duration and intensity with GammaCore compared to sham.
A preventive cluster headache study showed that weekly attack frequency decreased by 40% from baseline when GammaCore was added to standard of care therapy as a preventive treatment. There was a 57% decrease in the frequency of acute medication use when GammaCore was added.
What are the side effects of GammaCore?
Less than 5% of patients have side effects and include application site discomfort or tingling, muscle twitching, or dizziness. Treatment is often described as a deep vibration. There can be mild downward lip pulling during the treatment. GammaCore should not be used with an active implantable medical device, such as a pacemaker, hearing aid implant, or any implanted electronic device. It should be avoided in patients who have a metallic device such as a stent, bone plate, or bone screw implanted at or near their neck, are using another device at the same time (e.g., TENS Unit, muscle stimulator) or any portable electronic device (e.g., mobile phone).
How much does GammaCore cost and how do I get a GammaCore device?
Your doctor fills out a form on the GammaCore website and faxes it in. This begins the coordination between you and the company. The device itself is purchased and dispensed in 3 month treatment courses. Device capability allows for 30 two-minute stimulations each day for 3 months. 3 month refill RFID cards are prescribed for continued treatments, and the device is recharged with the refill card. Pricing and promotions are variable, depending on a number of factors and the company can provide more specific costs. GammaCore is fully covered by VA (Veterans Administration) health care benefits. Most FSA and HSA programs cover GammaCore and other neuromodulation devices.
@neuralgroover Neuromodulation Devices for Headaches Part 2: GammaCore Sapphire Vagus nerve stimulator for treatment of migraine, cluster headache, hemicrania continua, and paroxysmal hemicrania…! #GammaCore #GammaCoreSapphire #VagusNerve #Vagus #VagusNerveStimulator #VagusNerveStimulation #VagalNerve #VagalNerveStimulator #VagalNerveStimulation #ClusterHeadache #ClusterHeadaches #ClusterHeadacheTreatment #parasympatheticnervoussystem #parasympathetic #autonomicdysfunction #autonomicnervoussystem #dysautonomia #dysautonomiaawareness #POTS #potssyndrome #HemicraniaContinua #ParoxysmalHemicrania #Trigeminal #Neuromodulation #NeuromodulationForMigraine #Migraine #migrainerelieftok #ChronicMigraine #Migrainerelief #MigraineAbortive #migraineprevention #MigrainePreventive #Neurology #HeadacheSpecialist #Headachedoc #headachedoctor #headachemedicine #migrainesociety #migrainetok #migrainetiktok #neuroscience #nervoussystemregulation #nervoussystem #neurologist #brain #braindoctor #neurologistsoftiktok #headachetiktok #medical #medicaltiktok #medicaltiktoks #MigraineWarrior #headaches #Neuro #neurologytiktok #neurotok #headache #MigraineAwareness #foryou #fyp #tiktok #foryourpage #foryoupage #explore #trending #health #wellness #migrainesufferer #pain #chronicpain #medicalinnovation #blog #blogging #blogger #migraineblog #headacheblog #healthblog #medicalblog ♬ original sound – Virtual Headache Specialist🤯
What is Truvaga? What are the differences between GammaCore and Truvaga?
Truvaga is another type of vagus nerve stimulator, which is a consumer electronic device available without a prescription directly online. Notably, Truvaga is made by the same company that makes GammaCore, which is ElectroCore. The company makes no specific medical treatment claims since this specific device hasn’t been studied as robustly as GammaCore, nor has it received the same FDA clearances. However, Truvaga utilizes the same patented technology and frequency as GammaCore.
GammaCore is self contained with use controls on the device which makes it easier for headache patients to use. Truvaga is phone app enabled. You use buttons on the app to control the intensity of the Truvaga. Wellness customers tend to love the app features and are not challenged with app use by their pain like a headache patient often is.
How much is Truvaga?
There are 2 models available, Truvaga 350 ($299) and the newer model Truvaga Plus ($499). These costs are a one time purchase. The Truvaga 350 is good for 350 two-minute treatment sessions. The Truvaga Plus is good for unlimited two-minute treatment sessions. It is rechargeable and lasts about 3 years. Both models come with a 30 day risk free money back guarantee.
NERIVIO
What is Nerivio?
The Nerivio device is made by the company Theranica. It is a wireless remote electrical neuromodulation (REN) device wearable for the acute treatment of migraine applied to the upper-arm. It was FDA-cleared for the acute treatment of migraine in adults in May 2019 following this study. In October 2020, FDA clearance was extended to acute treatment of migraine in chronic migraine patients as well following this study. In January 2021, Nerivio received FDA clearance for acute migraine treatment for episodic or chronic migraine in patients 12 years and older with this study. In February 2023 it became FDA cleared for dual-use for both acute and preventive treatment of migraine with or without aura in patients 12 years or older. Furthermore, a 2023 study showed Nerivio to be a safe treatment option in pregnant women.
How does Nerivio work?
The device works through an app downloaded on your smartphone which controls the strength of stimulation. The device itself is an arm band that easily straps around the upper arm, and was recognized in TIME Magazine’s annual list of the 100 Best Inventions in 2019.
For abortive migraine treatment, Nerivio is applied within 60 minutes (preferably at onset) of a migraine headache or migraine aura, and stimulation is performed for 45 minutes. For preventive migraine treatment, a 45 minute Nerivio treatment is done every other day. Each device provides 16 treatments (as needed, or every other day for prevention). When the device is used up, it is recycled and a new refill device is sent.
Nerivio delivers electronic pulses into the skin to generate a proprietary “Conditioned Pain Modulation” response which helps to abort the migraine. Nerivio stimulates specific sensory nerves of the upper arm which normally sense pain. The stimulation from the device is not strong enough to actually trigger pain for the user, but the signal still travels to the brainstem. From here, it interferes and blocks the ongoing activated electrical circuitry of the migraine, and helps to abort it. It uses a proprietary stimulation signal which targets specific pain transmitting nerve fibers that disrupts the electrical activity of a migraine centrally from a remote location peripherally (on the arm). Nerivio is not the same as a TENS unit.
How well does Nerivio work?
A migraine abortive study showed that 67% of patients had significant pain relief at 2 hours, and 37.4% of patients achieved complete pain relief at 2 hours. Furthermore, 89.7% of patients studied avoided having to take other abortive medications when treating with Nerivio.
In a migraine abortive study leading to FDA clearance for adolescents 12 years and older in January 2021, 71% of patients had pain relief by 2 hours after Nerivio treatment, while 35% received complete pain relief. Pain relief and pain freedom were sustained for 24 hours in 90% of cases.
In the migraine preventive study, it was used every other day. Patients who used Nerivio had a mean reduction of 4 migraine days per month from baseline compared to a reduction of only 1.3 days in the placebo group.
What are the side effects of Nerivio?
Nerivio is often described as a vibrating sensation. Side effects may include a temporary sensation of warmth, local tingling, numbness in the arm, pain in the arm, or redness of the skin, although 96% of patients studied did not report any device related adverse events.
It is recommended to avoid in congestive heart failure, severe cardiac or cerebrovascular disease and uncontrolled epilepsy. It should not be used with certain medical devices such as a pacemaker or hearing aid implant. Using Nerivio with other implantable medical devices could potentially cause electric shock, electrical interference, or other injury. So it should not be used near any metallic implants.
How much does Nerivio cost and how do I get a Nerivio device?
Prescribing Process:
There is a prescription form found on the company’s website which is filled out and faxed in by your doctor. The prescriptions can also be sent electronically directly through most electronic medical record systems to ProCare Rx. The specialty pharmacy then contacts the patient to coordinate payment and delivery. Patients can also have an online visit with a healthcare provider via the company’s website to determine if Nerivio would be a treatment option, and they can prescribe it from there.
Pricing: (Pricing and promotions may vary, and updates can be found on the company’s website)
Nerivio is $49 for the first fill and refills are either $89 or $199 for 3 devices ($66 per device).
Insurance:
Nerivio is covered completely with $0 by VA (Veterans Administration) health care benefits. Insurance coverages have been increasing between various companies. Once prescribed, the ProCare Rx speciality pharmacy will be able to determine if there coverage options with your plan. Most FSA and HSA programs also cover Nerivio and other neuromodulation devices.
Current insurance coverages include:
Highmark BCBS Commercial Members
BCBS of Massachusetts will cover for Commercial and Medicare members (starting 2/1/25)
BCBS of North Dakota Commercial Members
Arizona Medicaid
Colorado Medicaid
DC Medicaid
New Jersey Medicaid
Ohio Medicaid
Virginia Medicaid
RELIVION MG
What is Relivion?
In March 2021, the company Neurolief received FDA clearance for its Relivion MG external combined occipital and trigeminal neurostimulation system (eCOT-NS) neuromodulation device for acute migraine treatment. Notably, it has also received recognition as an FDA breakthrough device for the treatment of major depression.
The Relivion MG device transmits treatment information to a patient app, where the smart technology can learn about your migraine. The Relivion MG system connects information about your migraine, treatments, lifestyle and environment to your doctor via a secure cloud database to analyze data and share insights with your doctor to help optimize care.
How does Relivion work?
This is a non-invasive, self-administered neuromodulation device used for the acute treatment of migraine attacks. The device is worn as a headset and simultaneously stimulates 6 peripheral nerve branches of the occipital (2 electrodes in the back of the head, 1 on each side over the greater occipital nerves) and trigeminal nerves (4 electrodes in the forehead, 2 on each side over the supraorbital and supratrochlear nerves).
Relivion MG is the first and only noninvasive neuromodulation device that stimulates the 2 main peripheral nerve pathways which are felt to influence migraine (trigeminal nerve pathway and occipital nerve pathway). This spread out stimulation allows for more stimulation to be delivered comfortably.
How well does Relivion work?
Clinical studies as an acute migraine treatment reported complete pain freedom at 2 hours in 46% of patients in the Relivion group vs. 11.8% of patients in the sham (placebo) group. 78% of those patients who became pain free at 2 hours had sustained pain freedom at 24 hours. Furthermore, 76% of patients in the Relivion group vs. 32% in the sham group had significant pain relief (so maybe not gone completely, but much less severe headache pain).
What are the side effects of Relivion?
In the RIME Study, a total of 12 adverse events were reported. All events were mild to moderate (ie scalp numbness, itching, skin irritation and redness) and were anticipated and self-resolved.
Conclusions
The 5 FDA cleared migraine neuromodulation devices above have been a great step forward in expanding treatment options for patients with migraine. These are Nerivio, GammaCore Vagus Nerve Stimulator, SAVI Dual, Cefaly, and Relivion MG. GammaCore is unique in that it is also FDA cleared for the treatment of cluster headache, hemicrania continua, and paroxysmal hemicrania. All have good supporting evidence. The device to try first depends on a few different things including cost, coverage options, and if you plan on using it for acute (abortive) treatment, preventive treatment, or both. If one type does not work, you can always consider a different device which works by a different mechanism.
The table at the bottom of this article is a summary of (some of) the comparison data between devices gathered from published studies and directly from the companies as well (check back periodically as updates are in progress). It’s important to keep in mind that the data in this table are not from head to head studies between devices, and there are different variables between the different studies which can influence results and outcomes.
| sTMS | Cefaly | GammaCore | Nerivio | |
|---|---|---|---|---|
| Acute Migraine | Yes | Yes | Yes | Yes |
| Preventive Migraine | Yes | Yes | Yes | No |
| Acute Cluster | No | No | Yes | No |
| Preventive Cluster | No | No | Yes | No |
| 1-hour migraine pain free | N/A | 32% (13% sham) | 21% (sham 10%) | N/A |
| 1-hour migraine pain relief | N/A | 79% (39% sham) | 35.8% (sham 24.4%) | N/A |
| 2-hour migraine pain free | 39% (sham 22%) | 17% (sham 7%) | 30.4% (sham 19.7%) | 37.4% (18.4% sham) |
| 2-hour migraine pain relief | N/A | 65% (sham 52%) | 40.8% (sham 27.6%) | 66.7% (38.8% sham) |
| Migraine preventive relief | 46% had > 50% decrease in monthly HA days (20% “statistically derived” placebo) and averaged 3 less migraine days/month | 29.7% decrease (sham 4.9%) 38.1% received at least 50% decrease in migraines (sham 12.12%) | 29% decrease (sham 18%) 35.8% decrease in patients with aura 33.6% received at least 50% decrease in migraines (sham 23.4%) | N/A |
IF YOU HAVE HEADACHE, MIGRAINE, OR FACIAL PAIN AND ARE LOOKING FOR ANSWERS ON ANYTHING RELATED TO IT, A HEADACHE SPECIALIST IS HERE TO HELP, FOR FREE!
FIRST, LET’S DECIDE WHERE TO START:
IF YOU HAVE AN EXISTING HEADACHE, MIGRAINE, OR FACIAL PAIN DIAGNOSIS AND ARE LOOKING FOR THE LATEST INFORMATION, HOT TOPICS, AND TREATMENT TIPS, VISIT OUR FREE BLOG OF HOT TOPICS AND HEADACHE TIPS HERE. THIS IS WHERE I WRITE AND CONDENSE A BROAD VARIETY OF COMMON AND COMPLEX MIGRAINE AND HEADACHE RELATED TOPICS INTO THE IMPORTANT FACTS AND HIGHLIGHTS YOU NEED TO KNOW, ALONG WITH PROVIDING FIRST HAND CLINICAL EXPERIENCE FROM THE PERSPECTIVE OF A HEADACHE SPECIALIST.
IF YOU DON’T HAVE AN EXISTING HEADACHE, MIGRAINE, OR FACIAL PAIN DIAGNOSIS AND ARE LOOKING FOR POSSIBLE TYPES OF HEADACHES OR FACIAL PAINS BASED ON YOUR SYMPTOMS, USE THE FREE HEADACHE AND FACIAL PAIN SYMPTOM CHECKER TOOL DEVELOPED BY A HEADACHE SPECIALIST NEUROLOGIST HERE!
IF YOU HAVE AN EXISTING HEADACHE, MIGRAINE, OR FACIAL PAIN DIAGNOSIS AND ARE LOOKING FOR FURTHER EDUCATION AND SELF-RESEARCH ON YOUR DIAGNOSIS, VISIT OUR FREE EDUCATION CENTER HERE.
FAQ
Neuromodulation is a type of therapy that involves electrical stimulation for the treatment of migraine. Neuromodulation acts directly on the nervous system within the targeted area. For migraines and cluster headaches, there are a few types of neuromodulatory devices available, including Nerivio, Cefaly, Relivion, Gammacore, and Spring TMS.
These devices have all been thoroughly and rigorously tested. Generally speaking, most patients will be able to safely use a neuromodulatory device for their cluster headaches or migraines. There are some people who should avoid using this type of technology, including people who have: metallic implants, pacemaker devices, aneurysm clips or coils, wearable defibrillators, and other medical devices. Your doctor will avoid prescribing neuromodulatory devices if they are not a good fit for this reason.
Neuromodulatory devices are a good fit for patients who are unable to tolerate some of the standard medications for migraines, or may not be able to take them because of medical contraindications. For example, people with heart and blood pressure issues might not be a good fit for triptans. In this case, they might be a good fit for a neuromodulatory device. On other occasions, the standard headache and migraine medications might not work effectively for patients. Neuromodulatory devices are prescribed when traditional prescription medications are not a good fit for the patient or simply won’t work.
Each different type of neuromodulatory device will have a “best fit” scenario based upon your headaches or migraines and the therapy that your doctor feels that you need. All of the different types of devices are approved and good for acute migraines. Some of them are also approved for migraine prevention. GammaCore is the best for acute cluster headaches, and the only device approved for cluster headaches. It is also used for migraine. If you are looking for a device that is affordable and highly effective, take a look at Nerivio if you have insurance or Medicaid/Medicare. This was the first device to receive an insurance benefit and will cost an initial copay of $10.
In terms of the length of treatment for using a neuromodulatory device, there isn’t a set length of time. Generally speaking, the patient will use the device for as long as treatment is effective or needed. However, each device works for different lengths of time:
- Nerivio: Each device is good for 12 different treatments.
- Cefaly: With Cefaly, the electrodes you will use will be good for about 20 sessions.
- Relivion: Each pack of six electrodes can be used for one treatment.
- Gammacore: This device uses an RFID treatment card which will let you use the device for 31 days.
- eNeura: The SpringTMS and sTMS mini are generally used by patients over an increment of 90 days. As with the Gammacore device, you will have a prescription, but in this instance, the prescription card is a SIM card.