
The Best Headache and Migraine Solutions Visualized
With frequent headaches and crushing migraines being all too common, we don’t need to tell you how debilitating they can be. That’s why we’re going

With frequent headaches and crushing migraines being all too common, we don’t need to tell you how debilitating they can be. That’s why we’re going

Yoga and meditation can help with fitness and mindfulness, but there are additional benefits. These activities can help you with migraines, headaches, and other types

I see patients in our headache center from all over the United States and from many other countries. Many patients travel hundreds of miles by
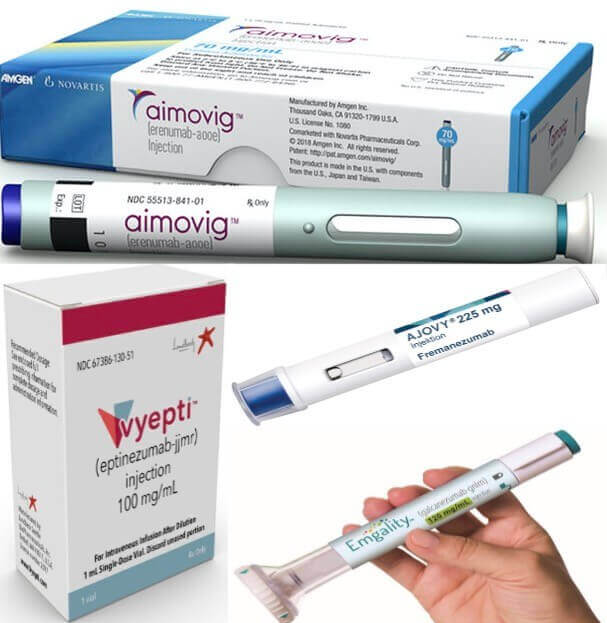
Which is the best CGRP monoclonal antibody (mAb) receptor antagonist for migraine prevention? Emgality vs. Ajovy, Ajovy vs. Vyepti, Aimovig vs. Ajovy, Aimovig vs. Emgality,

Migraine is a very disruptive disorder to have to deal with. It interferes with your family, work, financial, social, and educational lives. When the burden

The International Classification of Headache Disorders-3 (ICHD-3) classifies persistent aura without infarction (stroke) and migrainous infarction as two of the four reported complications of migraine,

Medical marijuana (medical cannabis) for treatment of migraine, headache, pain, and chronic pain has become an increasingly hot topic of interest. As states continue to

Migraine affects more than 10-12% of the world’s population, with approximately 1 billion migraineurs worldwide.1 There are 39 million migraineurs in the US, accounting for 12%

Nurtec vs. Ubrelvy vs. Zavzpret vs. Reyvow vs. Triptans. What are the differences and which is best? Imitrex (Sumatriptan) became available in 1992 and was

Chronic daily headache being endlessly fueled and driven by rebound headache (medication overuse headache or MOH) is one of the most common headache disorders that