Understanding CGRP Migraine Medications and Their Usage
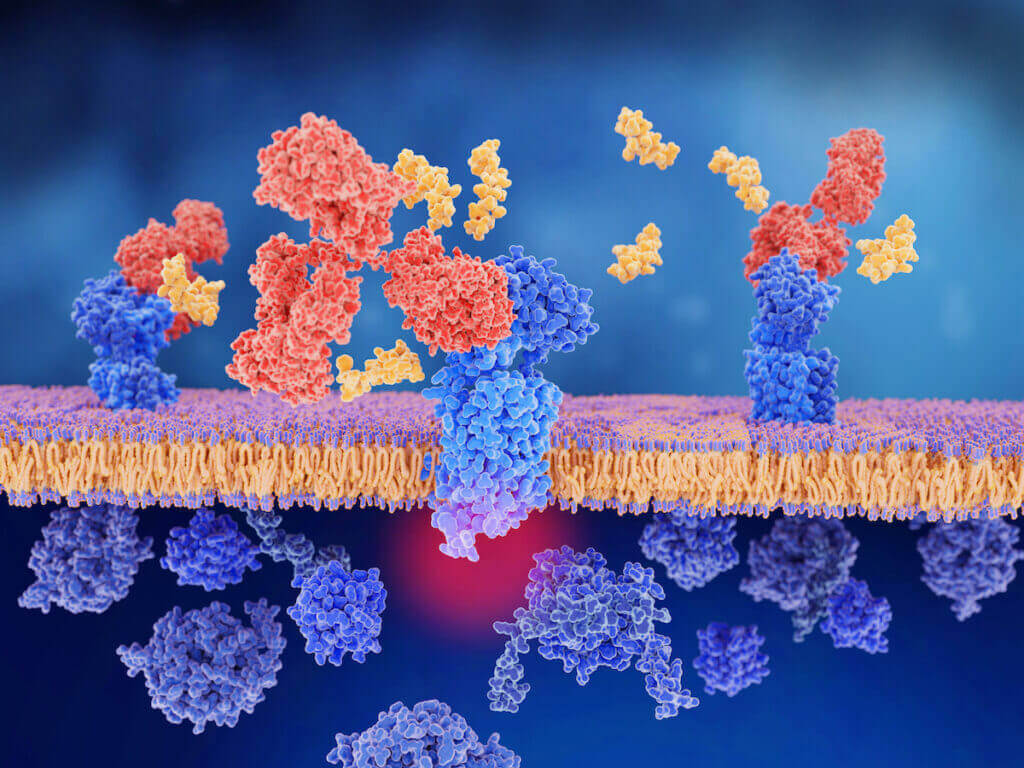
What is CGRP (Calcitonin Gene Related Peptide)? During a migraine attack, the trigeminal nerves release a variety of inflammatory proteins. One of the main proteins

What is CGRP (Calcitonin Gene Related Peptide)? During a migraine attack, the trigeminal nerves release a variety of inflammatory proteins. One of the main proteins

I see patients in our headache center from all over the United States and from many other countries. Many patients travel hundreds of miles by
Aimovig vs. Ajovy, Aimovig vs. Emgality, Aimovig vs Vyepti, Ajovy vs. Emgality, Emgality vs. Vyepti, Ajovy vs. Vyepti. So many questions. so many answers. Let’s

Migraine affects more than 10-12% of the world’s population, with approximately 1 billion migraineurs worldwide.1 There are 39 million migraineurs in the US, accounting for 12%

With all of these new treatments of acute medications for migraine headache such as the CGRP antagonists, as well as the standard triptans, how do

Imitrex vs. Maxalt, Zomig vs. Maxalt, Amerge vs. Relpax, Frova vs. Imitrex, Maxalt vs. Relpax, Zomig vs. Imitrex, Frova vs. Amerge, Imitrex vs. Treximet. Maxalt